The halogens are a group of non-metal elements found in group 17 of the periodic table. They include:
Fluorine (F): Highly reactive and used in toothpaste for dental health.
Chlorine (Cl): Commonly used as a disinfectant in swimming pools and water treatment.
Bromine (Br): Used in heated spas, in flame retardants and photography as it is more stable at higher temperatures than Chlorine.
Iodine (I): Essential for thyroid function and used as an antiseptic.
Astatine (At): Radioactive and rare, with limited practical applications.
Tennessine (Ts): Also radioactive and not widely studied.
The word “halogen” means “salt former,” as these elements react with metals to produce various salts. They exist in solid, liquid, and gaseous states at room temperature, making them unique in the periodic table
For our purposes, we are only interested in Chlorine (Cl) and Bromine (Br) as they apply to swimming pool sanitation.
They are :
OXIDISERS.:
What Are Oxidisers?
Oxidisers are chemicals that steal electrons from other compounds, breaking them down into their base components. They play a crucial role in pool water treatment.
Unlike sanitisers (which target pathogens like bacteria and viruses), oxidisers focus on destroying organic contaminants.
Typical bather contaminants come from hair spray, suntan lotion, body oil and perspiration.
Destroy micro-organisims, (very small individuals, each composed of mutually independent parts which work together to develop and support life)
Typical micro-organisims are bacteria, algae, fungi, and viruses. These again introduced by bathers and external factors.
Chlorine:
Description: Chlorine is the most popular pool sanitiser. It inactivates and kills harmful pathogens like E. coli, making the water safe for swimming.
Forms:
Liquid Chlorine, Chlorine tablets, Chlorine Powder (Granular Chlorine), or Chlorine gas (Chlorine gas is not commonly used in swimming pools, and is extremely hazardous).
All added directly to the pool water based on chemical test results.
Bromine:
Description: Bromine is an alternative sanitiser. It works similarly to chlorine, but is more stable at higher temperatures (ideal for Spas).
Chlorine Disassociation
CHLORINE ADDED TO WATER.
PH of water 7.6
+ pH
39.0 % --------------> 61.0%
HOCL <-------------- (H+) + (OCL-)
- pH
Hypochlorous Acid Hydrogen Ion Hypochlorite Ion
Equilibrium Reaction:
The dissociation of HOCl can be represented as an equilibrium reaction:
HOCl⇌H⁺+OCl⁻
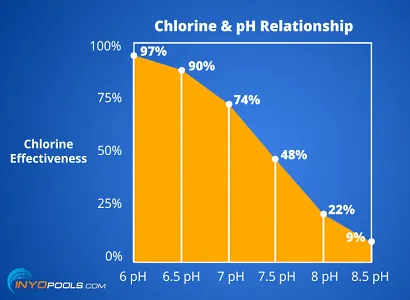
The balance between HOCl and OCl⁻ depends on the pH level:
At lower pH (more acidic conditions), there’s more HOCl (active form).
As pH increases, the proportion of OCl⁻ (less active form) rises
TEST KIT DPD NO1 OR R0001 / R0002 free chlorine (FC) READING OF
2.0 ppm
TEST KIT DPD No 3 OR R0003 total chlorine (TC) READING OF
3.2 ppm
The following calculation for combined chlorine is as follows
CC=TC-FC
1.2=3.2-2.0
COMBINED CHLORINE READING OF 1.2 PPM IS IN EXCESS OF ALLOWABLE.
MONOCHLOAMINES, DICHLORAMINES OR TRICHLORAMINES MAY BE PRESENT IN WATER.
Chlorine Residual
Chlorine residual refers to the amount of chlorine remaining in your pool water after it has reacted with organic matter, such as sweat, urine, and other contaminants. This residual chlorine is crucial for keeping your pool clean and free of harmful bacteria, viruses, and algae.
There are three main types of chlorine to understand:
Free Chlorine: This is the amount of chlorine available to sanitise your pool. It’s effective but can be unstable.
Combined Chlorine: Also known as chloramines, this is chlorine that has already reacted with contaminants. It’s less effective and can cause that characteristic chlorine smell.
Total Chlorine: This is the sum of free chlorine and combined chlorine in your pool.
Maintaining the right levels of chlorine residual is essential for effective pool sanitation. The ideal range for free chlorine in swimming pools is typically between 2.0 to 4.0 ppm (parts per million).
Effects of Temperature on Free Chlorine
Temperature has a significant impact on the effectiveness and stability of free chlorine in swimming pools. Here are the key points to consider:
Increased Chlorine Demand:
Warmer Water: As the temperature rises, the demand for chlorine increases. This is because higher temperatures promote the growth of bacteria and algae, which chlorine needs to kill.
Rule of Thumb:
For every 12°C increase above 27°C, the amount of chlorine needed to maintain adequate levels can double.
Faster Chlorine Degradation:
Chemical Reactions: Higher temperatures accelerate chemical reactions, including the breakdown of chlorine. This means chlorine will work faster but also get used up more quickly.
UV Exposure: Warm weather often coincides with more sunlight, which can further degrade chlorine unless stabilized by cyanuric acid.
Increased Organic Load:
More Swimmers: Warmer temperatures typically mean more pool usage, leading to higher levels of contaminants like sweat, sunscreen, and body oils. These increase the chlorine demand as well.
Practical Tips for Managing Chlorine Levels:
Regular Testing: Frequently test your pool water to monitor chlorine levels, especially during warmer months.
Adjust Chlorine Dosage: Increase the amount of chlorine added to the pool as temperatures rise to maintain effective sanitization.
Use Stabilizers: Consider using cyanuric acid to protect chlorine from UV degradation, especially in outdoor pools.
Maintaining the right chlorine levels is crucial for keeping your pool water clean and safe.
Effects of Rain on Free Chlorine
Yes, rain can affect chlorine levels in swimming pools. Heavy rainfall can dilute the chlorine in your pool, reducing its effectiveness in killing bacteria and algae. Additionally, rainwater often carries contaminants like dirt and debris into the pool, which can further disrupt the chemical balance.
It’s important to test and adjust your pool’s chlorine levels after heavy rain to ensure the water remains safe and clean. Do you
CHLORINE RESIDUALS
FREE AVAILABLE CHLORINE TEST. (FAC)
The chlorine residual present when a DPD 1 tablet or R-0001 + R-0002 liquid test is conducted. This test measures only the free chlorine in the water and indicates the sum of the HOCL and OCL species.
COMBINED CHLORINE TEST. (CC)
This test, DPD 3 or R0003 indicates any chloramines formed by the reaction of free chlorine with ammonia wastes from swimmers. Chloramines are combined chlorine and give the typical chlorine odour noticed at indoor pools.
The combined chlorine test is done on top of the FAC sample.
To calculate the combined chlorine figure, it is CC – FAC = CC
TOTAL CHLORINE (TC)
Total chlorine is not a test as such, but is a mathematical expression.
FAC + CC = TC
The effects of Urea on Chlorine
Commonly introduced into swimming pools through sweat and urine, has a significant impact on free chlorine levels.
Here’s how it affects pool chemistry:
Chemical Reactions
Formation of Chloramines: Urea reacts with free chlorine to form chloramines, which are compounds that can cause eye and skin irritation, as well as a strong chlorine odor.
Slow Oxidation:
The oxidation of urea by free chlorine is a slow process, leading to the formation of transient ammonia chloramines (e.g., di- and trichloramine).
Impact on Chlorine Effectiveness:
Chlorine Consumption: The reaction between urea and chlorine consumes free chlorine, reducing its availability for disinfecting the pool.
Increased Chlorine Demand: As urea levels increase, more chlorine is needed to maintain effective sanitization, leading to higher maintenance costs and efforts.
Health and Safety Concerns
Chloramines: These compounds can cause respiratory issues and discomfort for swimmers, especially in indoor pools where ventilation is limited.
Hygienic Risks: Urea can serve as a nutrient for bacteria and algae, potentially leading to increased microbial growth if not properly managed.
Management Tips
Regular Testing: Frequently test your pool water for urea and chloramine levels to ensure they remain within safe limits.
Proper Chlorination: Maintain adequate chlorine levels to ensure effective oxidation of urea and other nitrogen-containing compounds.
Good Hygiene Practices: Encourage swimmers to shower before entering the pool to reduce the introduction of urea and other contaminants.
Managing urea levels is crucial for maintaining a clean and safe swimming environment.
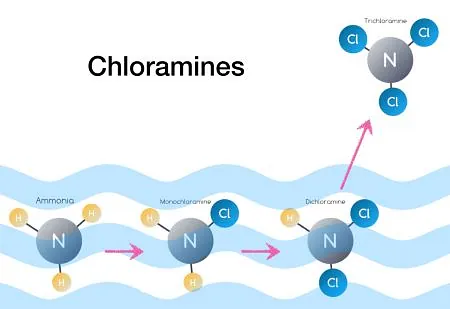
Types of chloramines:
Monochloramines in swimming pools are primarily caused by the reaction of free chlorine with nitrogen-containing compounds, such as sweat and urine, brought into the pool by swimmers.
Here are some key points:
Chlorine and Amine Reaction:
In swimming pools, chloramines are formed by the reaction of free chlorine with amine groups present in organic substances, mainly those biological in origin (e.g., urea in sweat and urine).
Ammonia and Chlorine Reaction:
As soon as ammonia enters a chlorinated pool, it reacts with hydrogen ions to quickly form ammonium ion. The ammonium ion reacts with hypochlorous acid to form monochloramines.
Chloramine Buildup:
Chloramines can build up in the water, which means they can build up in the air if there is not enough fresh air surrounding pools and other places people swim in chlorinated water.
Air Movement:
Three things cause the buildup of chloramines in the air:
1) Disturbing the water’s surface (for example, when swimmers move in the water or the water is sprayed through aquatic features),
2) Limiting movement of fresh air over the water’s surface, and
3) Using air handling systems to limit the amount of fresh air brought into the swimming area and limit the amount of air polluted with chloramines exhausted out of the swimming area.
Dichloramines in swimming pools are formed through a series of chemical reactions involving chlorine and nitrogen-containing compounds, such as those found in sweat, urine, and other organic substances introduced into the pool by swimmers.
Here are some key points:
Ammonia and Chlorine Reaction: When ammonia enters a chlorinated pool, it reacts with hydrogen ions to quickly form ammonium ion.
Formation of Monochloramines: The ammonium ion then reacts with hypochlorous acid to form monochloramines.
Formation of Dichloramines: Monochloramine reacts with free chlorine to form dichloramines.
Dichloramines are less stable than monochloramines and can rapidly decompose. They can cause unpleasant odors and irritation to swimmers. They also contribute to the familiar “chlorine smell” often associated with swimming pools. Therefore, it’s important to manage and control the levels of dichloramines in swimming pools to ensure a safe and pleasant swimming environment
Trichloramines in swimming pools are formed through a series of chemical reactions involving chlorine and nitrogen-containing compounds, such as those found in sweat, urine, and other organic substances introduced into the pool by swimmers.
Here are some key points:
Ammonia and Chlorine Reaction: When ammonia enters a chlorinated pool, it reacts with hydrogen ions to quickly form ammonium ion.
Formation of Monochloramines:
The ammonium ion then reacts with hypochlorous acid to form monochloramines.
Formation of Dichloramines:
Monochloramine reacts with free chlorine to form dichloramines.
Formation of Trichloramines:
Dichloramine reacts with free chlorine to form trichloramines.
Conditions that determine production and air levels of Trichloramines are believed to depend on the degree of water chlorination, contamination of water by nitrogen-containing compounds (which depends on the number of bathers, as well as their behaviour and hygiene), water temperature, water circulation and ventilation.
Trichloramines are an eye and airway irritant, but are also considered to be a possible cause of asthma in children.
To prevent or remove chloramines:
Minimise contaminants:
Encourage swimmers not to enter the water with diarrhoea, use the toilet before swimming, and avoid peeing or pooping in the water.
Rinse off:
Require swimmers to rinse off before getting into the water.
Optimise air handling systems:
Ensure proper ventilation to reduce chloramine accumulation while keeping heating costs down.
How to remove Chloramines:
Chloramines in swimming pools can cause that infamous chlorine smell, red eyes, and skin irritation. To remove them, consider the following methods:
- Super-Chlorination: With a stable pH (around 7.2), add enough liquid chlorine to reach approximately 10 ppm. This high level of free chlorine breaks apart ammonia-chlorine bonds, allowing ammonia or nitrogen to gas off the pool surface.
- Non-Chlorine Shock (MPS): Add a non-chlorine shock to the water.
- MPS helps oxidise chloramines.
- Ultraviolet (UV) Light: UV systems, commonly used in pharmaceutical industries, can break apart chloramine compounds when exposed to specific wavelengths.
Breakpoint Chlorination
Breakpoint chlorination is a critical concept in pool maintenance. It’s the point at which the disinfection demand has been met, and all undesirable contaminants in the pool have been oxidised. Think of it as a tipping point where oxidation is complete, and further additions of shock chlorine or other oxidisers become unnecessary.
Here are the key points about breakpoint chlorination:
Chloramines Removal: Breakpoint chlorination ensures that free chlorine levels are sufficient to fully remove chloramines (combined chlorine) from the pool water. Chloramines occur when free chlorine molecules attach to nitrogen or ammonia, rendering them ineffective. Removing chloramines is essential for water quality.
Threshold Level: Pool owners aim for a threshold level of free chlorine that achieves complete chloramine removal. If you fall short of this threshold, some chloramines and contaminants will persist. If you overshoot, you’ve used more oxidiser than necessary.
Formula: The generally accepted formula for breakpoint chlorination is to add 10 times the level of chloramines (as tested by a DPD test kit).
For example:
If your tested combined chlorine (CC) level is 0.5 ppm, add enough shock to reach 5.0 ppm.
If CC is 1.2 ppm, shock the pool to a level of 12.0 ppm.
Chlorine Types: The amount of chlorine needed depends on the type used:
2lts of 12% bleach adds 6.2 ppm of free chlorine.
500grms of 56% dichlor adds 6.6 ppm of free chlorine.
500grms of 65% calcium hypochlorite (Cal Hypo) adds 7.7 ppm of free chlorine.
Cyanuric Acid: If your pool contains cyanuric acid (used to protect chlorine from UV degradation), adjust the breakpoint calculation. Cyanuric acid limits chlorine activity, but requires less chlorine overall.
Remember, it’s better to add slightly more shock than needed to ensure thorough chloramine removal.
Types of chlorine and alternate sanitisers
Some important information:
The chlorine's described above needs to be addressed:
Calcium Hypochlorite contains calcium, so prolonged use will result in an increase in calcium hardness
When trichlor is used 0.6 ppm of cyanuric acid is added to the water for each ppm of available chlorine added.
Dichlor is 57% cyanuric acid; for each ppm of chlorine added, 0.9 ppm of cyanuric acid is added to the water.
The adjacent products are:
High Strength Non-Chlorine Shock Treatment:
Insta-Boost and Oxy-Boost is a powerful shock treatment that helps maintain water quality by eliminating combined chlorine levels.
Fast Dissolving Oxygen Compound:
The products dissolve rapidly in water, making it convenient for application.
It contains a minimum oxygen content of 13%.
Oxidises Organic Contaminants:
Insta-Boost & Oxy-Boost oxidises organic impurities, helping to keep your water clean and clear.
Application and Dosage:
In salt or chlorine-sanitised pools:Use 500 grams of Insta-Boost & Oxy-Boost to treat 50,000 litres of water.
For fountains:Use 25 grams to treat 5,000 litres of water.
Follow the dosage instructions for non-chlorine sanitising systems.
Important Note:
Insta-Boost & Oxy-Boost are not a sanitisers and do not replace the need for a registered sanitising agent.
Note - The Oxidising agent differ in both products. One is an AstralPool product Oxy-Boost, the other Insta-Boost is a Lo-Chlor product
Chlorine versus Salt Chlorination
Both chlorine and saltwater pools have their own advantages and disadvantages. Here’s a quick comparison to help you inform a customer which might be best for them:
Chlorine Pools
Pros:
Effective Sanitization: Chlorine is a powerful disinfectant that effectively kills bacteria and algae.
Lower Initial Cost: Setting up a chlorine pool is generally less expensive than a saltwater pool.
Easier to Adjust: You can quickly adjust chlorine levels by adding more chlorine.
Cons:
Chemical Handling: Requires regular handling and storage of chlorine chemicals.
Skin and Eye Irritation: Higher chlorine levels can cause irritation to the skin and eyes.
Maintenance: Requires frequent monitoring and maintenance to keep chlorine levels balanced.
Saltwater Pools
Pros:
Softer Water: The water in saltwater pools is gentler on the skin and eyes.