TOTAL ALKALINITY T.A.
Total Alkalinity (TA), also known as pool alkalinity, is a crucial aspect of swimming pool chemistry. It measures your pool water’s ability to resist changes in pH.
This works by absorbing acids in the water, which prevents those acids from lowering your pH level.
This is why total alkalinity is often referred to as a pH buffer, as it “shields” your pH from substances that would normally lower it.
Total alkalinity is important because it helps maintain a stable pH level in your pool. The ideal pH level for your pool water is slightly basic, somewhere between 7.2 to 7.8.
This is where chlorine works best without making the water too harsh on your skin, hair, and eyes.
Without total alkalinity acting as a buffer, your pH could fall too low, causing the water to become corrosive to your pool surfaces and equipment.
Conversely, if the pH drifts too high, it can lead to calcium scaling.
The substances that make up your total alkalinity include carbonates, bicarbonates, hydroxides, and cyanurates1. These are often introduced to the water through products that help you adjust pH and alkalinity, as well as chlorine stabilisers.
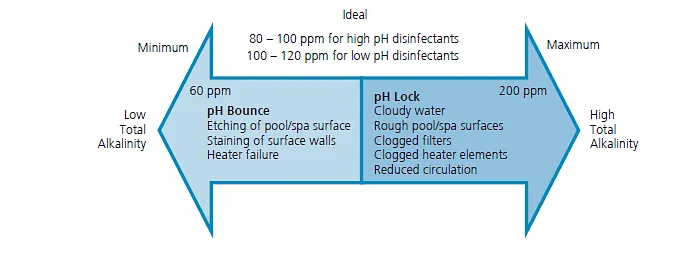
The ideal total alkalinity level for swimming pools is between 80 and 120 parts per million (ppm)134. When total alkalinity is too low, pH is unstable and may oscillate.
Conversely, when total alkalinity is too high, the buffering effect may cause pH to rise, diluting the sanitising efficacy of free chlorine.
Heavy or prolonged rain events can indeed affect the total alkalinity levels in swimming pools. Rainwater typically has a very low total alkalinity, close to zero. When it rains heavily, the rainwater dilutes the pool water, which can lower the total alkalinity levels. This reduction can make the water more acidic, potentially leading to corrosive conditions that can damage pool surfaces and equipment.
To adjust total alkalinity, you can raise it with buffer (Sodium bicarbonate) and lower it with sodium bisulfate or muriatic acid.
Regular testing and adjustment of total alkalinity are essential for maintaining a healthy and safe swimming pool environment.
pH Bounce
pH Bounce describes the rapid and cyclic fluctuation in the pH level of pool water between acidic and basic conditions. This occurs when the water lacks sufficient buffering capacity.
Here's an explanation:
High pH: A high pH level indicates that the water is too alkaline, which can lead to cloudy water or scaling on pool surfaces. To correct this, you would add an acid, such as muriatic acid or dry acid, to lower the pH.
Low pH: On the other hand, if the pH level is too low, the water becomes corrosive, which may damage the pool's walls, floors, and equipment. To counteract this, you would add a base, like sodium bicarbonate or soda ash, to increase the pH to the desired level.
Buffering Capacity: To prevent pH bounce, it's important to increase the water's alkalinity, which serves as a buffer to mitigate the impact of adding acids and bases. The goal is to keep alkalinity within the range of 60 - 180 ppm to stabilize pH levels.
It's essential to maintain the correct pH and alkalinity for balanced pool water. If alkalinity falls below 80 ppm, pH bounce can occur, resulting in unpredictable pH levels.
Soda Ash versus Sodium Bicarbonate
pH Level:
Soda ash (sodium carbonate) is a highly alkaline substance with a pH level between 11.3 and 11.71. Minimally raises alkalinity, making it suitable when you need a pH bump without significantly affecting total alkalinity (if it’s already in the proper range).
pH Level:
Buffer (sodium bicarbonate) has a lower pH level of 8. Commonly used to increase pH and total alkalinity.




